UPDATE Case 16-2011--- A 67 Year-Old Man with Recurrent Prostate Cancer
Matthew R. Smith, M.D., Ph.D., Anthony L. Zietman, M.D., Joel S. Finkelstein, M.D., and Chin-Lee Wu, M.D., Ph.D.
N Engl J Med 2011; 364:2044-2051May 26, 2011
N Engl J Med 2011; 364:2044-2051May 26, 2011
Dr. Philip J. Saylor (Medical Oncology): A 67-year-old man was seen in the multidisciplinary genitourinary cancer clinic at this hospital because of recurrent prostate cancer.
Approximately 18 months earlier, needle biopsies of the prostate were performed because of an elevated level of prostate-specific antigen (PSA). A diagnosis of adenocarcinoma of the prostate was made, with a Gleason score of 7 (grade 3 plus grade 4). (The Gleason score is the sum of the two most prevalent histologic grades in a prostate tumor, each of which is rated on a scale of 1 to 5, with 5 being the most cytologically aggressive.)
Two months later, radical retropubic prostatectomy and bilateral lymph-node sampling were performed. Pathological examination of the tissue revealed adenocarcinoma with a Gleason score of 8 (4 plus 4) in the right and left posterior quadrants of the prostate (with capsular penetration), extending to within 0.1 cm of the inked resection margins. The pathological stage was pT3N0. Three months after prostatectomy, the level of PSA was undetectable (0.5 ng per milliliter; reference value for men after prostatectomy, <0.5). Adjuvant external-beam radiation therapy was administered. On routine follow-up at the cancer center at this hospital 18 months after prostatectomy, the PSA level was 17.2 ng per milliliter.
The patient reported feeling well, with no urinary or bowel symptoms. He had a history of recurrent diverticulitis. Medications included acetylsalicylic acid daily. He had no known drug allergies. He was a widower and worked in an office. He drank alcohol occasionally and did not smoke or use illicit drugs. His mother had died at 94 years of age, his father at 60 years after a myocardial infarction, and his brother at 70 years, of leukemia; there was no other family history of cancer. A bone scan revealed marked right thoracic spinal scoliosis and nonspecific focal uptake of technetium-99m–labeled methylene diphosphonate within the right iliac crest and left distal radius. The location and pattern of the uptake were consistent with degenerative joint disease. A radiograph of the pelvis and a computed tomographic (CT) scan of the abdomen and pelvis showed no evidence of recurrent or metastatic carcinoma. A management decision was made.
DISCUSSION OF MANAGEMENT
Dr. Anthony L. Zietman
Prostate cancer was diagnosed in this patient after screening showed an elevated PSA level; a radical prostatectomy was performed. May we review the pathological specimens?
PATHOLOGICAL DISCUSSION
Dr. Chin-Lee Wu
Specimens from four core needle biopsies of the prostate were obtained with the use of ultrasonographic guidance
Adenocarcinoma was identified in all four cores, occupying 25 to 65% of the tissue. The Gleason score for the biopsy specimens in this patient was 7 (3 plus 4). Gleason grades are assigned according to a scale of 1 (most differentiated architecture) to 5 (least differentiated architecture).1-3 Gleason grade is associated with cancer-specific survival. Prostate cancers are known to be heterogeneous, often having more than one grade. The Gleason score, which is the sum of the grades of the most abundant architectural pattern and the second most abundant pattern, takes into account this tumor heterogeneity.2,3
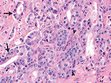
In the specimen from the radical prostatectomy, extensive cancer was identified in both lobes of the gland.
Figure 2 Radical Prostatectomy Specimen (Hematoxylin and Eosin).
The final Gleason score of 8 (4 plus 4) indicated that the tumor grade had been underestimated on the biopsy specimens. Because of tumor heterogeneity and sampling error, prostate cancer is upgraded at prostatectomy in approximately 40% of patients. In this patient's prostatectomy specimen, cancer invaded the perineural space, which is an indication of cancer aggression. There was extraprostatic tumor extension, and the tumor was close to the resection margins (1 mm from the margin in some areas). The seminal vesicles and bilateral obturator lymph nodes were uninvolved by tumor. The final American Joint Committee on Cancer tumor–node–metastasis pathological stage was pT3aN0Mx.
In the specimen from the radical prostatectomy, extensive cancer was identified in both lobes of the gland.
Figure 2 Radical Prostatectomy Specimen (Hematoxylin and Eosin).
The final Gleason score of 8 (4 plus 4) indicated that the tumor grade had been underestimated on the biopsy specimens. Because of tumor heterogeneity and sampling error, prostate cancer is upgraded at prostatectomy in approximately 40% of patients. In this patient's prostatectomy specimen, cancer invaded the perineural space, which is an indication of cancer aggression. There was extraprostatic tumor extension, and the tumor was close to the resection margins (1 mm from the margin in some areas). The seminal vesicles and bilateral obturator lymph nodes were uninvolved by tumor. The final American Joint Committee on Cancer tumor–node–metastasis pathological stage was pT3aN0Mx.
DISCUSSION OF MANAGEMENT
Dr. Zietman
The final pathology report is rich with important prognostic information. For men with organ-confined, low-volume, low-grade (Gleason score of 6 or less) cancer, the risk of relapse is very small. For men with features that carry a higher risk of relapse, additional therapy may be necessary.
Further local therapy, such as radiation, would be appropriate only if this patient were at considerable risk for local recurrence, particularly if he had positive surgical margins. If this patient had high-grade disease (Gleason score of 8, 9, or 10) or disease that had extended into the seminal vesicle or spread to local lymph nodes, he would be at greater risk of distant recurrence than of local recurrence.
For such patients, radiation therapy is inappropriate, and systemic therapy with androgen deprivation makes more sense. The results of this patient's pathological examination sat in a “gray zone.” His tumor was very close to the margin but was not positive, and he had high-grade disease but no spread to the seminal vesicles or lymph nodes. Partly on the basis of his long life expectancy, we recommended adjuvant radiation therapy. In contrast to androgen-deprivation therapy, which is very unlikely to be curative after surgery, radiation gave him a second chance for cure. The timing of radiation therapy after surgery is under question. Some argue to start therapy early, at a time when the cancer is at its lowest volume and, perhaps, most likely to be cured (adjuvant therapy).
There are many precedents for adjuvant therapy in oncology, most notably for the treatment of breast cancer. Alternatively, one can take advantage of the ability of PSA to act as a sensitive tumor marker and reserve radiation for when the PSA level rises, if ever (salvage therapy). Salvage therapy allows some men (including those who never have a recurrence and those who relapse rapidly with metastatic disease) to avoid radiation therapy and its risks. This patient elected early adjuvant radiation therapy. The results of three randomized trials that were reported after his treatment appear to support the decision.
One trial showed an advantage in freedom from biochemical failure (a rising level of PSA) for men receiving immediate rather than salvage radiation.4 A second study, with a longer follow-up, showed a reduction in clinical failure.5 A third study, with a follow-up period of more than 15 years, showed an improvement in survival rates.6 The number of men with stage pT3 disease who would need to be treated with adjuvant radiation therapy to prevent one death at 10 years was approximately 12. Adjuvant radiation carries risks, which were explained to this patient. Since the bladder neck, retrovesical space, and vesicourethral anastomosis are the likely sites of residual cancer, they all need treatment. As a result, men have temporary urinary frequency and urgency and may also have bowel urgency.
Urinary incontinence beyond that resulting from surgery is unusual. However, the rate of recovery of sexual potency is significantly lower after adjuvant radiation than after surgery alone. Serious complications are rare, since the radiation fields are small and since total radiation doses can be kept relatively low, owing to the microscopical volume of the cancer. This patient received 61.2 Gy in 34 daily fractions, with no short-term complications. Dr. Matthew R. Smith: Eighteen months after prostatectomy, the serum PSA level of this patient was elevated, at 17.2 ng per milliliter, but a physical examination and radiographic imaging showed no evidence of local or distant disease.
Although the rising serum PSA level after prostatectomy and radiation therapy in this patient heralds the development of locally recurrent or metastatic disease, the natural history of the recurrence of such “PSA only” disease is variable. In a retrospective study of men with a rising PSA level after prostatectomy who were followed without treatment, for example, the median time from the first postoperative elevation in the PSA level to detectable metastatic disease was 8 years.7 A higher Gleason score, a shorter interval from surgery to the first elevation in the PSA level, and a shorter time to the doubling of the PSA level were associated with a shorter time to metastases. For men such as this patient, with a Gleason score of 8, 9, or 10 and less than a 2-year interval from prostatectomy to the first rise in the PSA level, the probability of metastases within 5 years after the first rise in the PSA level is 60% without additional therapy. In contrast, the probability of metastases within 5 years was only 18% for men with a Gleason score of 5 to 7 and an interval greater than 2 years from prostatectomy to the first elevation of the PSA level.
Androgen-deprivation therapy (ADT), either by means of bilateral orchiectomies or the administration of a gonadotropin-releasing hormone (GnRH) agonist or antagonist, is the mainstay of treatment for metastatic prostate cancer and for the recurrence of high-risk PSA-only disease.8 The intended effect of ADT is severe hypogonadism. ADT lowers gonadal steroid levels to the prepubertal range. In men with bone metastases, ADT is associated with an objective response rate of greater than 80% and a response duration of approximately 2 years. Long-term neoadjuvant treatment with a GnRH agonist improves the rates of disease-free and overall survival in men receiving external-beam radiation therapy for locally advanced or high-risk nonmetastatic prostate cancer.9,10 Adjuvant therapy with a GnRH agonist also improves the survival rate in men with node-positive disease after prostatectomy.11 The optimal timing of ADT for men with PSA-only disease recurrence, such as this patient, is controversial. In a systematic review of immediate ADT versus ADT that was deferred until clinical progression for men with locally advanced or metastatic prostate cancer, early ADT was associated with greater 10-year rates of overall survival.12 The number who would need to be treated to prevent one death at 10 years was approximately 25.
Although these data suggest a survival benefit of early ADT for this patient, the potential advantage must be balanced against the greater incidence of adverse events associated with this treatment than with deferred treatment. ADT has a variety of adverse effects related to hypogonadism, including loss of libido, vasomotor flushing, fatigue, anemia, and osteoporosis. 8 ADT also is associated with a variety of adverse metabolic effects.13 ADT decreases muscle mass (by approximately 3% during the first year of treatment) and increases fat mass (by approximately 10% during the first year of treatment). ADT increases fasting plasma insulin levels and decreases insulin sensitivity. ADT also increases the levels of serum triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. Consistent with these adverse metabolic effects, ADT has been linked to increased risks of diabetes and cardiovascular disease.14-16 Most (but not all) population-based studies have reported a significant link between ADT and myocardial infarction.14,15 Most studies, including analyses of five large, randomized, controlled trials from the Radiation Therapy Oncology Group and the European Organisation for Research and Treatment of Cancer, reported no significant relationship between ADT and death from cardiovascular causes.17 After considering the potential benefits and harms, I recommended prompt ADT for this patient. He was treated with leuprolide acetate. We also prescribed bicalutamide, a nonsteroidal antiandrogen, for 1 month, to prevent the potential flare associated with the initial administration of a GnRH agonist.
The patient was advised to follow a healthy diet and exercise regularly to mitigate the potential effects of ADT on body composition. Because of the risk of bone loss that is associated with ADT, the patient's bone mineral density was measured by dual-energy x-ray absorptiometry (DXA). As is often the case in older men, the DXA scan of his spine was unusable because of degenerative arthritic changes. The bone mineral density of the femoral neck and total hip were 0.72 g per square centimeter (T score [defined as the number of standard deviations by which a patient's bone mineral density differs from that of a healthy 30-year-old of the same sex and ethnic background], −1.6) and 0.94 g per square centimeter (T score, −0.6), respectively. The serum PSA level declined to undetectable levels within 3 months after the start of treatment.
Skeletal Complications Associated with ADT Dr. Joel S. Finkelstein: Because this man with prostate cancer may receive therapy with a GnRH agonist for many years, the consequences of ADT, including osteoporosis, are a potentially important clinical problem. Whereas severe hypogonadism will develop in all women who live until midlife, because of natural menopause, severe hypogonadism rarely occurs naturally in men, and ADT is now the most common cause of severe male hypogonadism. Because this patient's bone mineral density at baseline was reasonably good, he was initially treated with calcium and vitamin D alone. In general, the bone mineral density of the lumbar spine declines by 3 to 4% in the first year of ADT.18,19 Decreases in the bone mineral density of the proximal femur are more moderate. In several studies, the bone mineral density of the shaft of the radius declined more than did the bone mineral density of the spine or proximal femur.20 During the next 3 years (Table 1TABLE 1 Results of Bone Mineral Density Testing. the bone mineral density of the femoral neck and total hip in this patient declined by approximately 10%, to 0.65 g per square centimeter (T score, −2.10) and 0.84 g per square centimeter (T score, −1.2), respectively. These changes are typical of GnRH agonist–induced bone loss in men and are associated with a doubling of the fracture risk.21,22 What should be done now? Randomized, controlled trials involving at least eight antiresorptive agents have assessed whether treatment-related bone loss can be prevented. Bisphosphonates, including pamidronate, zoledronic acid, and alendronate,18,23,24 and selective estrogen-receptor modulators, including raloxifene and toremifene,25,26 prevent bone loss during ADT.
Denosumab, a human monoclonal antibody against the receptor activator of nuclear factor-κB ligand, increased bone mineral density and reduced the incidence of vertebral fractures in men with prostate cancer who received ADT in a randomized, controlled trial.27 In patients like this one, with T scores that are greater than −2.5 and no history of osteoporotic fractures, many experts would recommend treatment with calcium and vitamin D and then assess the fracture risk with the Fracture Risk Assessment Tool (FRAX) (www.shef.ac.uk/FRAX)28 to decide whether to add an antiresorptive agent. This patient's 10-year estimated risks of any major osteoporotic fracture and hip fracture were 10% and 3.9%, respectively. Most current guidelines recommend antiresorptive therapy if these 10-year risks exceed 20% and 3%, respectively. On the basis of the available information, I would recommend antiresorptive treatment for this patient. There is strong evidence of the efficacy of denosumab, but because of its high cost I would begin with oral alendronate; if this results in adverse events, I would change to either intravenous zoledronic acid or denosumab. I would also consider measuring the patient's bone mineral density with a technique that can assess sites that are enriched in trabecular bone but are unaffected by degenerative changes, such as quantitative CT of the lumbar spine or DXA of the ultradistal radius.
Follow-up
Dr. Smith:
After 3 years, leuprolide was discontinued at the patient's request because of vasomotor flushing, loss of libido, fatigue, and subjective weakness. At the time of the discontinuation, the level of serum PSA was undetectable. After the discontinuation of leuprolide, the serum testosterone level promptly returned to a normal level and the patient's symptoms of vasomotor flushing, fatigue, and weakness improved. The administration of zoledronic acid every 6 months was begun for the treatment of osteoporosis. Two and a half years after the discontinuation of ADT, the serum PSA level was markedly elevated. Subsequent imaging studies showed multiple bone metastases. The patient was retreated with leuprolide and remained on continuous treatment. Three months later, results of a repeat PSA test showed an undetectable level. Two years after the patient resumed ADT, the disease progressed (castration-resistant disease), as evidenced by rising serum levels of PSA and repeat imaging studies that showed new bone metastases
Further local therapy, such as radiation, would be appropriate only if this patient were at considerable risk for local recurrence, particularly if he had positive surgical margins. If this patient had high-grade disease (Gleason score of 8, 9, or 10) or disease that had extended into the seminal vesicle or spread to local lymph nodes, he would be at greater risk of distant recurrence than of local recurrence.
For such patients, radiation therapy is inappropriate, and systemic therapy with androgen deprivation makes more sense. The results of this patient's pathological examination sat in a “gray zone.” His tumor was very close to the margin but was not positive, and he had high-grade disease but no spread to the seminal vesicles or lymph nodes. Partly on the basis of his long life expectancy, we recommended adjuvant radiation therapy. In contrast to androgen-deprivation therapy, which is very unlikely to be curative after surgery, radiation gave him a second chance for cure. The timing of radiation therapy after surgery is under question. Some argue to start therapy early, at a time when the cancer is at its lowest volume and, perhaps, most likely to be cured (adjuvant therapy).
There are many precedents for adjuvant therapy in oncology, most notably for the treatment of breast cancer. Alternatively, one can take advantage of the ability of PSA to act as a sensitive tumor marker and reserve radiation for when the PSA level rises, if ever (salvage therapy). Salvage therapy allows some men (including those who never have a recurrence and those who relapse rapidly with metastatic disease) to avoid radiation therapy and its risks. This patient elected early adjuvant radiation therapy. The results of three randomized trials that were reported after his treatment appear to support the decision.
One trial showed an advantage in freedom from biochemical failure (a rising level of PSA) for men receiving immediate rather than salvage radiation.4 A second study, with a longer follow-up, showed a reduction in clinical failure.5 A third study, with a follow-up period of more than 15 years, showed an improvement in survival rates.6 The number of men with stage pT3 disease who would need to be treated with adjuvant radiation therapy to prevent one death at 10 years was approximately 12. Adjuvant radiation carries risks, which were explained to this patient. Since the bladder neck, retrovesical space, and vesicourethral anastomosis are the likely sites of residual cancer, they all need treatment. As a result, men have temporary urinary frequency and urgency and may also have bowel urgency.
Urinary incontinence beyond that resulting from surgery is unusual. However, the rate of recovery of sexual potency is significantly lower after adjuvant radiation than after surgery alone. Serious complications are rare, since the radiation fields are small and since total radiation doses can be kept relatively low, owing to the microscopical volume of the cancer. This patient received 61.2 Gy in 34 daily fractions, with no short-term complications. Dr. Matthew R. Smith: Eighteen months after prostatectomy, the serum PSA level of this patient was elevated, at 17.2 ng per milliliter, but a physical examination and radiographic imaging showed no evidence of local or distant disease.
Although the rising serum PSA level after prostatectomy and radiation therapy in this patient heralds the development of locally recurrent or metastatic disease, the natural history of the recurrence of such “PSA only” disease is variable. In a retrospective study of men with a rising PSA level after prostatectomy who were followed without treatment, for example, the median time from the first postoperative elevation in the PSA level to detectable metastatic disease was 8 years.7 A higher Gleason score, a shorter interval from surgery to the first elevation in the PSA level, and a shorter time to the doubling of the PSA level were associated with a shorter time to metastases. For men such as this patient, with a Gleason score of 8, 9, or 10 and less than a 2-year interval from prostatectomy to the first rise in the PSA level, the probability of metastases within 5 years after the first rise in the PSA level is 60% without additional therapy. In contrast, the probability of metastases within 5 years was only 18% for men with a Gleason score of 5 to 7 and an interval greater than 2 years from prostatectomy to the first elevation of the PSA level.
Androgen-deprivation therapy (ADT), either by means of bilateral orchiectomies or the administration of a gonadotropin-releasing hormone (GnRH) agonist or antagonist, is the mainstay of treatment for metastatic prostate cancer and for the recurrence of high-risk PSA-only disease.8 The intended effect of ADT is severe hypogonadism. ADT lowers gonadal steroid levels to the prepubertal range. In men with bone metastases, ADT is associated with an objective response rate of greater than 80% and a response duration of approximately 2 years. Long-term neoadjuvant treatment with a GnRH agonist improves the rates of disease-free and overall survival in men receiving external-beam radiation therapy for locally advanced or high-risk nonmetastatic prostate cancer.9,10 Adjuvant therapy with a GnRH agonist also improves the survival rate in men with node-positive disease after prostatectomy.11 The optimal timing of ADT for men with PSA-only disease recurrence, such as this patient, is controversial. In a systematic review of immediate ADT versus ADT that was deferred until clinical progression for men with locally advanced or metastatic prostate cancer, early ADT was associated with greater 10-year rates of overall survival.12 The number who would need to be treated to prevent one death at 10 years was approximately 25.
Although these data suggest a survival benefit of early ADT for this patient, the potential advantage must be balanced against the greater incidence of adverse events associated with this treatment than with deferred treatment. ADT has a variety of adverse effects related to hypogonadism, including loss of libido, vasomotor flushing, fatigue, anemia, and osteoporosis. 8 ADT also is associated with a variety of adverse metabolic effects.13 ADT decreases muscle mass (by approximately 3% during the first year of treatment) and increases fat mass (by approximately 10% during the first year of treatment). ADT increases fasting plasma insulin levels and decreases insulin sensitivity. ADT also increases the levels of serum triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. Consistent with these adverse metabolic effects, ADT has been linked to increased risks of diabetes and cardiovascular disease.14-16 Most (but not all) population-based studies have reported a significant link between ADT and myocardial infarction.14,15 Most studies, including analyses of five large, randomized, controlled trials from the Radiation Therapy Oncology Group and the European Organisation for Research and Treatment of Cancer, reported no significant relationship between ADT and death from cardiovascular causes.17 After considering the potential benefits and harms, I recommended prompt ADT for this patient. He was treated with leuprolide acetate. We also prescribed bicalutamide, a nonsteroidal antiandrogen, for 1 month, to prevent the potential flare associated with the initial administration of a GnRH agonist.
The patient was advised to follow a healthy diet and exercise regularly to mitigate the potential effects of ADT on body composition. Because of the risk of bone loss that is associated with ADT, the patient's bone mineral density was measured by dual-energy x-ray absorptiometry (DXA). As is often the case in older men, the DXA scan of his spine was unusable because of degenerative arthritic changes. The bone mineral density of the femoral neck and total hip were 0.72 g per square centimeter (T score [defined as the number of standard deviations by which a patient's bone mineral density differs from that of a healthy 30-year-old of the same sex and ethnic background], −1.6) and 0.94 g per square centimeter (T score, −0.6), respectively. The serum PSA level declined to undetectable levels within 3 months after the start of treatment.
Skeletal Complications Associated with ADT Dr. Joel S. Finkelstein: Because this man with prostate cancer may receive therapy with a GnRH agonist for many years, the consequences of ADT, including osteoporosis, are a potentially important clinical problem. Whereas severe hypogonadism will develop in all women who live until midlife, because of natural menopause, severe hypogonadism rarely occurs naturally in men, and ADT is now the most common cause of severe male hypogonadism. Because this patient's bone mineral density at baseline was reasonably good, he was initially treated with calcium and vitamin D alone. In general, the bone mineral density of the lumbar spine declines by 3 to 4% in the first year of ADT.18,19 Decreases in the bone mineral density of the proximal femur are more moderate. In several studies, the bone mineral density of the shaft of the radius declined more than did the bone mineral density of the spine or proximal femur.20 During the next 3 years (Table 1TABLE 1 Results of Bone Mineral Density Testing. the bone mineral density of the femoral neck and total hip in this patient declined by approximately 10%, to 0.65 g per square centimeter (T score, −2.10) and 0.84 g per square centimeter (T score, −1.2), respectively. These changes are typical of GnRH agonist–induced bone loss in men and are associated with a doubling of the fracture risk.21,22 What should be done now? Randomized, controlled trials involving at least eight antiresorptive agents have assessed whether treatment-related bone loss can be prevented. Bisphosphonates, including pamidronate, zoledronic acid, and alendronate,18,23,24 and selective estrogen-receptor modulators, including raloxifene and toremifene,25,26 prevent bone loss during ADT.
Denosumab, a human monoclonal antibody against the receptor activator of nuclear factor-κB ligand, increased bone mineral density and reduced the incidence of vertebral fractures in men with prostate cancer who received ADT in a randomized, controlled trial.27 In patients like this one, with T scores that are greater than −2.5 and no history of osteoporotic fractures, many experts would recommend treatment with calcium and vitamin D and then assess the fracture risk with the Fracture Risk Assessment Tool (FRAX) (www.shef.ac.uk/FRAX)28 to decide whether to add an antiresorptive agent. This patient's 10-year estimated risks of any major osteoporotic fracture and hip fracture were 10% and 3.9%, respectively. Most current guidelines recommend antiresorptive therapy if these 10-year risks exceed 20% and 3%, respectively. On the basis of the available information, I would recommend antiresorptive treatment for this patient. There is strong evidence of the efficacy of denosumab, but because of its high cost I would begin with oral alendronate; if this results in adverse events, I would change to either intravenous zoledronic acid or denosumab. I would also consider measuring the patient's bone mineral density with a technique that can assess sites that are enriched in trabecular bone but are unaffected by degenerative changes, such as quantitative CT of the lumbar spine or DXA of the ultradistal radius.
Follow-up
Dr. Smith:
After 3 years, leuprolide was discontinued at the patient's request because of vasomotor flushing, loss of libido, fatigue, and subjective weakness. At the time of the discontinuation, the level of serum PSA was undetectable. After the discontinuation of leuprolide, the serum testosterone level promptly returned to a normal level and the patient's symptoms of vasomotor flushing, fatigue, and weakness improved. The administration of zoledronic acid every 6 months was begun for the treatment of osteoporosis. Two and a half years after the discontinuation of ADT, the serum PSA level was markedly elevated. Subsequent imaging studies showed multiple bone metastases. The patient was retreated with leuprolide and remained on continuous treatment. Three months later, results of a repeat PSA test showed an undetectable level. Two years after the patient resumed ADT, the disease progressed (castration-resistant disease), as evidenced by rising serum levels of PSA and repeat imaging studies that showed new bone metastases
(Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Since that time, he has been treated with zoledronic acid monthly to reduce the risk of disease-related skeletal complications. He received investigational treatment with sunitinib, an oral, small-molecule, receptor tyrosine kinase inhibitor; his best response was stable disease. He was then treated with docetaxel chemotherapy for 10 months; his best response was stable disease. He then enrolled in a randomized, phase 3 clinical trial (ClinicalTrials.gov number, NCT00638690) comparing abiraterone plus prednisone with placebo plus prednisone for castration-resistant metastatic prostate cancer; he had stable disease after 2 years. When the trial was unblinded, we learned that the patient was taking placebo plus prednisone, but he declined crossover to open-label abiraterone; the results of this trial are published in this issue of the Journal. At his last follow-up, 13 years after the diagnosis of prostate cancer, the patient was taking narcotic analgesic agents as needed for pain at multiple skeletal sites. The serum PSA level was 50.6 ng per milliliter. A bone scan showed extensive skeletal uptake of technetium-99m–labeled methylene diphosphonate, a feature that is consistent with widespread bone metastases. Dr. Nancy Lee Harris (Pathology): Is there a role for up-front ADT in men with a diagnosis of high-risk disease? Dr. Smith: Studies in the adjuvant setting provide the most compelling evidence of the efficacy of ADT for patients with prostate cancer.
Long-term adjuvant ADT is associated with improved overall survival rates in men with node-positive disease after prostatectomy. Adjuvant ADT improves survival rates in men receiving primary radiation therapy for intermediate-risk and high-risk prostate cancer. In men with high-risk disease, the treatment effect is large; the number who would need to be treated to prevent one death at 8 years was approximately 5. Long-term adjuvant ADT is associated with an improved overall survival rate in men who have node-positive disease after prostatectomy. It is notable that our patient's prostatectomy specimen did not show lymph-node metastases.
A Physician: Are the adverse effects of orchiectomy similar to those associated with therapy with a GnRH agonist? Dr. Smith: For a variety of reasons, orchiectomies are an uncommon form of ADT in the United States. On the basis of the available evidence, I believe that the adverse effects of ADT result from hypogonadism and that the adverse effects are similar for both orchiectomy and therapy with a GnRH agonist.
A Physician: You have described adverse metabolic effects, increased lipid levels, and osteoporosis. Do these occur in distinct populations or in the same people?
Dr. Smith: We believe that all patients with prostate cancer who undergo ADT are at risk for these adverse metabolic effects. However, osteoporosis, diabetes, or cardiovascular disease will not develop in all men because the baseline risks for these conditions and the magnitude of treatment-related metabolic alterations are variable.
A Physician: Is there any treatment for the muscle loss? Dr. Smith: We advise our patients about the importance of good nutrition and exercise. ADT is associated with fatigue, so many men find it particularly difficult to exercise. There are no established drug therapies to prevent muscle loss in survivors of prostate cancer. Prevention and treatment of sarcopenia remain important unmet medical needs.
A Physician: There is some evidence that bisphosphonates prevent bone metastases in women with breast cancer. Do bisphosphonates or other drugs prevent bone metastases in men with prostate cancer?
Dr. Smith: In some, but not all, studies involving women with nonmetastatic breast cancer, adjuvant therapy with a bisphosphonate decreased the risk of bone metastases. In contrast, published studies involving patients with prostate cancer have shown no benefit of bisphosphonates or other drugs for the prevention of metastases. Notably, the failure of metastasis-prevention studies in prostate cancer may be explained by limitations of study design, including the enrollment of subjects at low risk for progression. An ongoing global clinical trial (NCT00286091) is investigating the use of denosumab for the prevention of metastases in men who have castration-resistant nonmetastatic prostate cancer and who are at high risk for metastases on the basis of PSA levels, PSA velocity, or both.
Long-term adjuvant ADT is associated with improved overall survival rates in men with node-positive disease after prostatectomy. Adjuvant ADT improves survival rates in men receiving primary radiation therapy for intermediate-risk and high-risk prostate cancer. In men with high-risk disease, the treatment effect is large; the number who would need to be treated to prevent one death at 8 years was approximately 5. Long-term adjuvant ADT is associated with an improved overall survival rate in men who have node-positive disease after prostatectomy. It is notable that our patient's prostatectomy specimen did not show lymph-node metastases.
A Physician: Are the adverse effects of orchiectomy similar to those associated with therapy with a GnRH agonist? Dr. Smith: For a variety of reasons, orchiectomies are an uncommon form of ADT in the United States. On the basis of the available evidence, I believe that the adverse effects of ADT result from hypogonadism and that the adverse effects are similar for both orchiectomy and therapy with a GnRH agonist.
A Physician: You have described adverse metabolic effects, increased lipid levels, and osteoporosis. Do these occur in distinct populations or in the same people?
Dr. Smith: We believe that all patients with prostate cancer who undergo ADT are at risk for these adverse metabolic effects. However, osteoporosis, diabetes, or cardiovascular disease will not develop in all men because the baseline risks for these conditions and the magnitude of treatment-related metabolic alterations are variable.
A Physician: Is there any treatment for the muscle loss? Dr. Smith: We advise our patients about the importance of good nutrition and exercise. ADT is associated with fatigue, so many men find it particularly difficult to exercise. There are no established drug therapies to prevent muscle loss in survivors of prostate cancer. Prevention and treatment of sarcopenia remain important unmet medical needs.
A Physician: There is some evidence that bisphosphonates prevent bone metastases in women with breast cancer. Do bisphosphonates or other drugs prevent bone metastases in men with prostate cancer?
Dr. Smith: In some, but not all, studies involving women with nonmetastatic breast cancer, adjuvant therapy with a bisphosphonate decreased the risk of bone metastases. In contrast, published studies involving patients with prostate cancer have shown no benefit of bisphosphonates or other drugs for the prevention of metastases. Notably, the failure of metastasis-prevention studies in prostate cancer may be explained by limitations of study design, including the enrollment of subjects at low risk for progression. An ongoing global clinical trial (NCT00286091) is investigating the use of denosumab for the prevention of metastases in men who have castration-resistant nonmetastatic prostate cancer and who are at high risk for metastases on the basis of PSA levels, PSA velocity, or both.
ANATOMICAL DIAGNOSIS
Prostatic adenocarcinoma, Gleason score 8 (grade 4 plus grade 4), American Joint Committee on Cancer tumor–node–metastasis pathological stage pT3aN0Mx, recurrent after prostatectomy and radiation treatment. This case was discussed at the Massachusetts General Hospital Cancer Center Grand Rounds. Dr. Finkelstein reports receiving grant support from Solvay Pharmaceuticals and AstraZeneca Pharmaceuticals. Dr. Wu reports having patents pending, together with Massachusetts General Hospital, for testing related to the diagnosis of prostate cancer. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. SOURCE INFORMATION From the Divisions of Hematology–Oncology (M.R.S.) and Endocrinology (J.S.F.), and the Departments of Radiation Oncology (A.L.Z.) and Pathology (C.-L.W.), Massachusetts General Hospital; and the Departments of Medicine (M.R.S., J.S.F.), Radiation Oncology (A.L.Z.), and Pathology (C.-L.W.), Harvard Medical School — both in Boston.
REFERENCES
1Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966;50:125-128
Medline
2Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64
Web of Science | Medline
3Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 2005;216:34-63
CrossRef | Medline
4Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 2009;27:2924-2930
CrossRef | Web of Science | Medline
5Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised con-trolled trial (EORTC trial 22911). Lancet 2005;366:572-578
CrossRef | Web of Science | Medline
6Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009;181:956-962
CrossRef | Web of Science | Medline
7Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-1597
CrossRef | Web of Science | Medline
8Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238-244
CrossRef | Web of Science | Medline
9Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997;337:295-300
Full Text | Web of Science | Medline
10Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol 1997;15:1013-1021
Web of Science | Medline
11Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 1999;341:1781-1788
Full Text | Web of Science | Medline
12Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev 2002;1:CD003506-CD003506
Medline
13Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 2009;181:1998-2008
CrossRef | Web of Science | Medline
14Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448-4456
CrossRef | Web of Science | Medline
15Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009;27:3452-3458
CrossRef | Web of Science | Medline
16Lage MJ, Barber BL, Markus RA. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 2007;70:1104-1108
CrossRef | Web of Science | Medline
17Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 2010;121:833-840
CrossRef | Web of Science | Medline
18Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med 2001;345:948-955
Full Text | Web of Science | Medline
19Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005;90:6410-6417
CrossRef | Web of Science | Medline
20Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab 2002;87:3656-3661
CrossRef | Web of Science | Medline
21Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154-164
Full Text | Web of Science | Medline
22Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005;23:7897-7903
CrossRef | Web of Science | Medline
23Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003;169:2008-2012
CrossRef | Web of Science | Medline
24Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med 2007;146:416-424
Web of Science | Medline
25Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 2004;89:3841-3846
CrossRef | Web of Science | Medline
26Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol 2010;184:1316-1321[Erratum, J Urol 2011;185:365.]
CrossRef | Web of Science | Medline
27Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745-755
Full Text | Web of Science | Medline
28Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008;19:385-397
CrossRef | Web of Science | Medline
REFERENCES
1Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966;50:125-128
Medline
2Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64
Web of Science | Medline
3Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 2005;216:34-63
CrossRef | Medline
4Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol 2009;27:2924-2930
CrossRef | Web of Science | Medline
5Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised con-trolled trial (EORTC trial 22911). Lancet 2005;366:572-578
CrossRef | Web of Science | Medline
6Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009;181:956-962
CrossRef | Web of Science | Medline
7Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-1597
CrossRef | Web of Science | Medline
8Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238-244
CrossRef | Web of Science | Medline
9Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997;337:295-300
Full Text | Web of Science | Medline
10Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol 1997;15:1013-1021
Web of Science | Medline
11Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 1999;341:1781-1788
Full Text | Web of Science | Medline
12Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev 2002;1:CD003506-CD003506
Medline
13Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 2009;181:1998-2008
CrossRef | Web of Science | Medline
14Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448-4456
CrossRef | Web of Science | Medline
15Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009;27:3452-3458
CrossRef | Web of Science | Medline
16Lage MJ, Barber BL, Markus RA. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 2007;70:1104-1108
CrossRef | Web of Science | Medline
17Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 2010;121:833-840
CrossRef | Web of Science | Medline
18Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med 2001;345:948-955
Full Text | Web of Science | Medline
19Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005;90:6410-6417
CrossRef | Web of Science | Medline
20Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab 2002;87:3656-3661
CrossRef | Web of Science | Medline
21Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154-164
Full Text | Web of Science | Medline
22Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005;23:7897-7903
CrossRef | Web of Science | Medline
23Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003;169:2008-2012
CrossRef | Web of Science | Medline
24Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med 2007;146:416-424
Web of Science | Medline
25Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 2004;89:3841-3846
CrossRef | Web of Science | Medline
26Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol 2010;184:1316-1321[Erratum, J Urol 2011;185:365.]
CrossRef | Web of Science | Medline
27Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745-755
Full Text | Web of Science | Medline
28Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008;19:385-397
CrossRef | Web of Science | Medline



Thanks for sharing this informative information with us...
BalasHapusExcellent resource !! I have already bookmarked this page for more useful information !! Thank you !
BalasHapus